Global Warning Issued by WHO Against Three Indian Cough Syrups — Coldrif, Respifresh TR, and ReLife
Global Warning Issued by WHO Against Three Indian Cough Syrups — Coldrif, Respifresh TR, and ReLife
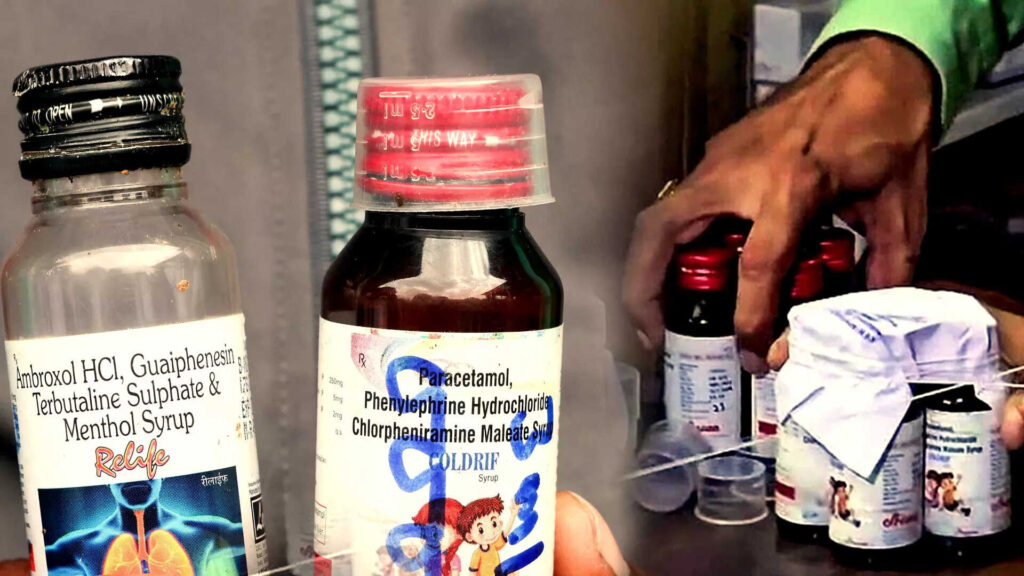
Mounting concerns have emerged on the global health front after the World Health Organization (WHO) issued a high-priority medical alert regarding three contaminated cough syrups manufactured in India. The syrups in question—Coldrif, Respifresh TR, and ReLife—are produced by Sresan Pharmaceutical, Rednex Pharmaceuticals, and Shape Pharma, respectively. WHO has confirmed that these products contain toxic substances that can cause severe harm, particularly in children, and has called for immediate international action.
The warning comes in the wake of at least 20 tragic child deaths reported in Madhya Pradesh, all under the age of five. These fatalities occurred within a short period during the first week of October, following the use of Coldrif syrup. Investigations carried out by India’s Central Drugs Standard Control Organisation (CDSCO) revealed that the children died of acute kidney failure, a condition triggered by the ingestion of diethylene glycol (DEG)—a dangerous industrial solvent.
Lab analysis of samples taken from the Tamil Nadu manufacturing facility responsible for Coldrif showed a shocking 48.6% weight/volume concentration of DEG, far exceeding any permissible limits. Exposure to DEG can result in symptoms such as lethargy, vomiting, abdominal pain, and dark-colored urine, rapidly progressing to kidney failure and death, especially in young children.
WHO’s global alert strongly advises individuals to immediately stop using any of the listed syrups. Regulatory bodies and healthcare professionals around the world have been urged to remain vigilant and report any cases involving these medicines. Special attention is being called to informal and unregulated markets, where such harmful products may enter circulation undetected.
Following the confirmation of contamination, Indian authorities have taken decisive action by banning the sale, distribution, and stockpiling of Coldrif and related products. WHO, meanwhile, is requesting national regulatory agencies across all countries to carry out targeted inspections and implement stricter surveillance, especially concerning oral liquid medicines originating from the same manufacturers—particularly those produced since December 2024.
Reports from the U.S. Food and Drug Administration (FDA) have confirmed that these toxic products have not entered the United States. However, WHO continues to advise extreme caution, stressing that countries should not assume immunity based on current trade records alone.
Healthcare providers are encouraged to report any side effects, adverse reactions, or lack of therapeutic response associated with these syrups to their local Pharmacovigilance Centres or National Regulatory Authorities. Prompt reporting could play a crucial role in stopping the further spread of these substandard and potentially deadly products.












