Rajasthan Tragedy: Cough Syrup Claims 2 Children’s Lives, 10 Fall Ill; Doctor Drinks Dose to Prove Safety, Collapses
Rajasthan Tragedy: Cough Syrup Claims 2 Children’s Lives, 10 Fall Ill; Doctor Drinks Dose to Prove Safety, Collapses
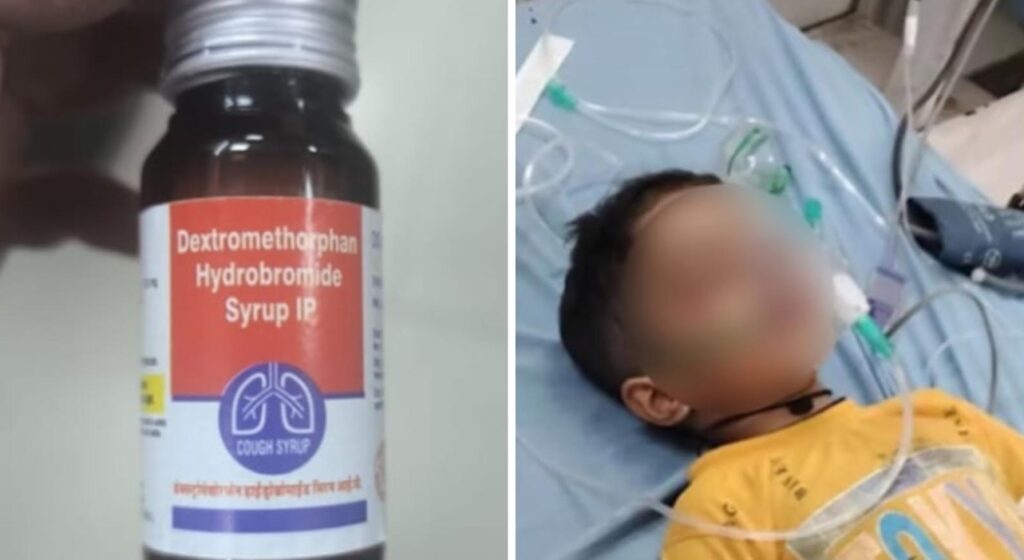
Shock and grief have spread across Rajasthan as a government-distributed cough syrup, manufactured by Kayson Pharma, has allegedly led to the deaths of two children and caused serious health issues in at least ten others. Alarming reports have surfaced from multiple districts over the past two weeks, prompting an urgent response from health authorities.
The syrup, containing dextromethorphan hydrobromide, was being dispensed through various government health centres. Trouble began when 5-year-old Nitish from Sikar district tragically passed away just hours after consuming a prescribed dose.
Nitish had been taken to the Community Health Centre (CHC) in Chirana for treatment of a routine cold and cough. He was given the syrup by his mother around 11:30 PM on Sunday. Later that night, he briefly woke up, asked for water, and returned to sleep. But by morning, he was unresponsive.
His family rushed him to the hospital, where he was declared dead on arrival.
“Nitish was perfectly fine during the day. He had even gone out to attend the Navratri celebration,” said his uncle Priyakant Sharma, as reported by NDTV. “He started coughing again late at night, so we gave him the syrup that was provided by the CHC. But in the morning, he wouldn’t wake up. We first took him back to the CHC, where the compounder asked us to go straight to the Sikar government hospital. He had taken the correct dose and was feeling okay before that.”
Soon after Nitish’s case, another family in Malha village, Bharatpur connected the same cough syrup to the death of their 2-year-old child, Samrat Jatav.
Samrat, along with his sister Sakshi and cousin Virat, had all taken the syrup after showing symptoms of illness. While Sakshi and Virat later regained consciousness—though with severe vomiting—Samrat sadly did not survive. He passed away at JK Lon Hospital in Jaipur.
“Three of my grandchildren took the same medicine. We had no idea it could be dangerous,” said Samrat’s grandmother Nehni Jatav. “Two of them eventually woke up, but I lost little Samrat. We only realised the medicine might be the cause after hearing about the boy from Sikar and others falling sick.”
In a separate and deeply troubling development from Bayana, a local doctor, Dr. Tarachand Yogi, attempted to prove the syrup’s safety after a parent complained her three-year-old fell ill. He drank a dose of the syrup himself and even gave some to an ambulance driver.
Shortly afterward, Dr. Yogi began feeling drowsy, stopped his car on the roadside, and lost consciousness for nearly eight hours. His family located him using his mobile phone. The ambulance driver also showed symptoms but recovered with medical help.
Meanwhile, eight more children—aged between 1 and 5 years—in Banswara district reportedly fell sick after consuming the same syrup last week. One 6-year-old was in critical condition initially, but later stabilised.
According to Dr. Pradyuman Jain, paediatrician at Mahatma Gandhi Government Hospital in Banswara, as cited by NDTV:
“The drug suspected of causing breathing issues and extreme drowsiness has been banned. In some cases, the reactions could be due to overdosing. Fortunately, most children have responded well to treatment, and even the seriously ill 6-year-old has now recovered.”
Following the spate of incidents, the Rajasthan government has acted swiftly, banning 22 specific batches of the syrup and halting its distribution statewide. Authorities confirmed that since July, over 1.33 lakh bottles of this syrup had been distributed, with 8,200 bottles still stored at SMS Hospital in Jaipur—now quarantined and not being dispensed.
All doctors across the state have been instructed to immediately stop prescribing the syrup, while samples have been sent for lab testing. The state’s supply relationship with Kayson Pharma has been suspended. It’s worth noting that the same pharmaceutical company faced regulatory action in 2023, when one of its products was blacklisted over quality concerns.
With investigations underway, and families mourning the unimaginable, the focus now turns to accountability, stricter testing, and ensuring such a devastating lapse never happens again.












