Multivalent meningococcal meningitis vaccine from Serum Institute of India achieves WHO prequalification
This milestone marks a key step forward in protecting African meningitis belt countries from deadly and debilitating meningitis epidemics
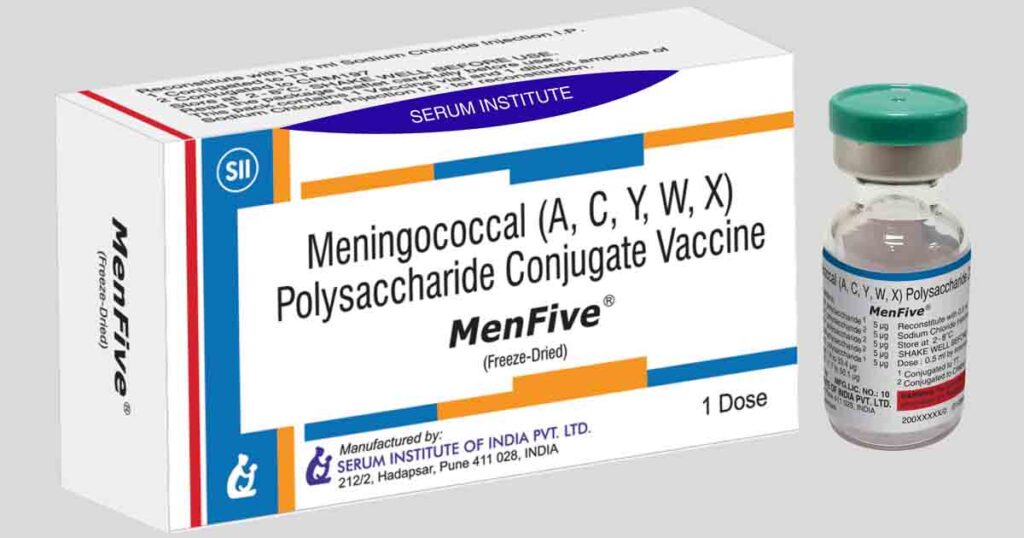
Pune: 12 July 2023 – MenFive®, the first conjugate vaccine to protect against the five predominant causes of meningococcal meningitis in Africa, has been prequalified by the World Health Organization (WHO). Developed through a 13-year collaboration between Serum Institute of India Pvt. Ltd. (SIIPL) and PATH, with crucial funding from the UK government’s Foreign, Commonwealth and Development Office, MenFive® protects against meningococcal serogroups A, C, W, Y, and X and is designed to eliminate annual meningitis outbreaks and epidemics in the African meningitis belt—a string of 26 countries from Senegal and The Gambia in the west to Ethiopia in the east. It is also the only vaccine that prevents meningitis caused by meningococcal group X, a pathogen increasingly implicated in meningitis outbreaks in Africa.
WHO prequalification—which ensures a vaccine meets strict international quality, safety, and efficacy standards—was supported by extensive clinical studies in The Gambia, India, and Mali that demonstrated a high level of safety and immunogenicity. Importantly, prequalification allows MenFive® to be procured by United Nations agencies and Gavi, The Vaccine Alliance.
Adar Poonawalla, CEO, Serum Institute of India, said, “MenFive® is a game-changer vaccine developed through a powerful 13-year collaboration between SIIPL, PATH, and vital support from the UK government, in the fight against meningococcal meningitis in Africa. As the first conjugate vaccine to safeguard against the five predominant causes of this deadly disease, MenFive® offers hope for a future free from annual outbreaks and epidemics in the African meningitis belt. It is a big moment as we, together, pave the way towards a healthier Africa, saving countless lives.”
“MenFive® is a much-required medical intervention that will be available at an extremely affordable price,” says Dr. Rajeev Dhere, Executive Director of SIIPL. “Making sure vaccines are available to those who need them most is a philosophy SIIPL has followed with all our products and continues to follow with MenFive®.”
“This landmark scientific achievement will have huge implications for improving public health,” says the UK’s International Development Minister Andrew Mitchell. “Having access to a new, affordable vaccine will save lives, prevent long-term illness, and move us closer to defeating meningitis by 2030. I am incredibly proud that the UK has supported PATH and the Serum Institute of India in this major achievement.”
Meningococcal meningitis is a bacterial infection that sets in rapidly and can kill within hours. It can cause severe brain damage and sepsis leading to limb amputation and is fatal in 50 percent of cases if untreated. Anyone can contract meningococcal meningitis but children under age five—especially infants—are likely to suffer the most severe effects.1
Polysaccharide vaccines have traditionally been used in response to African meningitis epidemics, but they have limitations. They only provide short-term protection, don’t promote herd immunity and are not generally effective in infants and children younger than 2 years of age. Conjugate vaccines provide better, longer lasting protection against meningococcal disease.
Multivalent meningococcal conjugate vaccines that protect against serogroup A, C, W, and Y have been available on the global market for decades, but they aren’t affordable for meningitis belt countries to include in their meningitis prevention strategies—leaving 450 million people at risk of death or severe disability due to meningococcal disease.
“The prequalification of MenFive® represents a turning point for the African meningitis belt and a step forward in the global effort to Defeat Meningitis by 2030,” says Dr. Bill Hausdorff, director of PATH’s meningitis vaccine development projects. “The introduction of new multivalent meningococcal conjugate vaccines is a key strategy for bacterial meningitis prevention and control. MenFive® is a critical addition to the toolbox that will save thousands of lives every year.”
“Meningococcal meningitis has long been a torment for meningitis belt countries,” says Dr. Nanthalile Mugala, PATH Africa Region Chief. “The 2010 introduction of meningococcal A vaccine—which eliminated meningitis A epidemics from the African meningitis belt—was a landmark achievement that showed freedom from meningococcal meningitis was possible. But it was only the beginning of the story. With MenFive®, we now have the potential to finally end all meningococcal meningitis epidemics in Africa, once and for all.”
MenFive® builds on the legacy of MenAfriVac®, SIIPL’s groundbreaking vaccine—developed in partnership with PATH and WHO—that eliminated serogroup A meningococcal meningitis outbreaks from the African meningitis belt following its 2010 introduction. MenFive® is designed to prevent not just death from meningitis, but also disability in survivors who would suffer lifelong social and economic consequences.
And, MenFive® is expected to provide an affordable new intervention with two highly impactful health outcomes:
1) broad, highly effective direct protection against invasive meningococcal disease; and
2) indirect “herd” protection (to unvaccinated people) by markedly reducing the meningococci bacteria in the nose and throat that is key to transmission.
MenFive® is approved by WHO for use in individuals 1 through 85 years of age and will initially be available for use in reactive vaccine campaigns for meningitis outbreaks. Discussions are currently underway among WHO, its partners, and affected countries as to the most effective strategy for controlling meningococcal meningitis with MenFive® through a combination of proactive vaccination campaigns and as a replacement for MenAfriVac® in the routine immunization schedule. Additionally, because MenFive® is the only vaccine that protects against meningococcal serogroup X, it may have potential for use in other regions of the world.
MenFive® is currently undergoing an additional Phase 3 study in healthy children between 9 and 15 months of age in Mali, to examine MenFive®’s safety and immunogenicity when administered alongside measles/rubella and yellow fever vaccine. The study is being conducted by the Infectious Diseases Clinical Research Consortium in collaboration with the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health. Studies like this could help expand the availability of MenFive® to ensure it protects as many people as possible and advance the goal of defeating meningococcal meningitis epidemics in Africa.
***
About PATH
PATH is a global nonprofit dedicated to achieving health equity. With more than 40 years of experience forging multisector partnerships, and with expertise in science, economics, technology, advocacy, and dozens of other specialties, PATH develops and scales up innovative solutions to the world’s most pressing health challenges. For more information, visit https://www.path.org/
About Serum Institute of India Pvt. Ltd.
Serum Institute of India Pvt. Ltd, is a global leader in vaccine manufacturing, dedicated to providing affordable vaccines worldwide. Present across 170+ countries, including the US, UK, and Europe, SII holds the distinction of being the world’s largest vaccine manufacturer. SII’s multifunctional production and one-of-the-largest facility in Manjri, Pune, with an annual capacity of 4 billion doses, has saved over 30 million lives over the years.
Founded in 1966, SII’s primary mission is to produce life-saving immunobiological drugs, with a particular emphasis on affordability and accessibility. Guided by a strong commitment to improving global health, the company has played a pivotal role in reducing the prices of essential vaccines, such as Diphtheria, Tetanus, Pertussis, HIB, BCG, r-Hepatitis B, Measles, Mumps, and Rubella. Notably, they are the manufacturers of ‘Pneumosil,’ the world’s most affordable PCV, and the first indigenous qHPV vaccine in India. Moreover, SII has been at the forefront of the global fight against COVID-19, delivering over 2 billion doses of the COVID-19 vaccine worldwide.
To further expand its global presence and ensure widespread vaccine availability, SII has established Serum Life Sciences Ltd, a subsidiary in the UK. Through relentless pursuit of innovation, SII continues to champion the cause of affordable vaccines, making a positive impact on the lives of millions worldwide. www.seruminstitute.com